Articles from SurGenTec

SurGenTec® has received additional FDA 510(k) clearance for OsteoFlo® HydroFiber™, its advanced synthetic bone graft, marking another key regulatory milestone. This expansion of indications now includes use as a bone void filler for the treatment of tumors, cysts, trauma, and osteomyelitis. This landmark clearance represents a significant advancement in the capabilities of SurGenTec®’s cutting-edge technology and highlights the company’s commitment to enhancing patient care in orthopedic and spine surgery.
By SurGenTec · Via Business Wire · July 22, 2025

SurGenTec®, a leading innovator in minimally invasive orthopedic and spine surgery solutions, is proud to announce the launch of its new TiLINK®-INSITE™ Sacroiliac Joint Fusion System. The system—now available in a sterile, surgery-ready kit—has already been successfully utilized in initial procedures performed across multiple U.S. sites, receiving strong endorsements from physicians.
By SurGenTec · Via Business Wire · July 15, 2025

SurGenTec proudly announces that its groundbreaking product, OsteoFlo HydroFiber, has received FDA 510(k) clearance, marking a significant milestone in the advancement of bone graft technology. OsteoFlo HydroFiber is now recognized as a stand-alone solution, equivalent to autograft for spine surgeries, including use in interbody fusion cages, disc spaces, and posterolateral fusions.
By SurGenTec · Via Business Wire · January 16, 2025

SurGenTec, an innovative medical device company specializing in orthopedic and spine technology, is thrilled to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its proprietary B-MAN Bone Marrow Aspirate Kit.
By SurGenTec · Via Business Wire · August 23, 2024

SurGenTec, a pioneering medical device company specializing in orthopedic and spine technologies, proudly announces the first implantations and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary OsteoFlo HydroPutty Synthetic Bone Graft.
By SurGenTec · Via Business Wire · March 7, 2024

SurGenTec, a privately held medical device company focusing on spine and orthopedic technologies, proudly announces FDA clearance for TiLink-P, a first-of-its-kind, minimally-invasive implant that offers hope for individuals suffering from chronic sacroiliac (SI) joint pain.
By SurGenTec · Via Business Wire · October 3, 2023
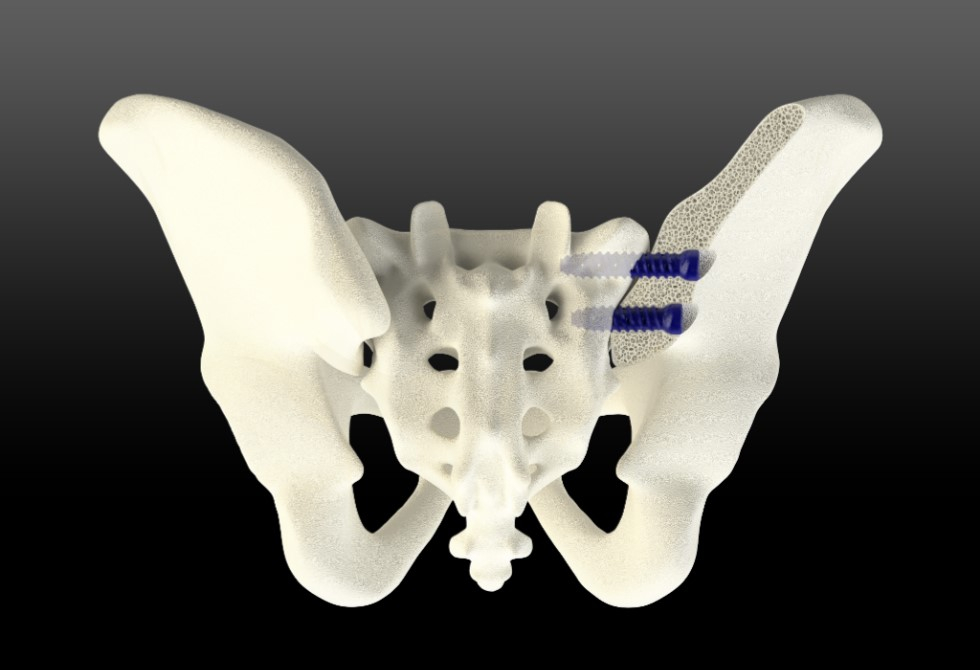
A leader in innovative medical devices, is proud to announce clearance from the U.S. Food and Drug Administration for their proprietary TiLink-L Sacroiliac Joint Fusion System. The SI joint implant may be implanted from a lateral or posterior/oblique approach and uses unique surface technology to optimize bone growth. This implant will be SurGenTec’s inaugural "First Implant in the Sacroiliac Family of Products."
By SurGenTec · Via Business Wire · June 8, 2023
