Articles from Mirvie

A new study sponsored by Mirvie, which is the first to evaluate U.S. Preventive Services Task Force (USPSTF) guidelines for preeclampsia risk and aspirin prescription in a single, nationally representative, prospective population, found while high-risk factors had sufficient value in estimating risk, there’s limited value for the moderate risk category - leading to nonspecific recommendations for aspirin use, a recognized prevention tool.
By Mirvie · Via Business Wire · July 17, 2025

Mirvie, a leader in uncovering the biology and utilizing data-driven approaches to improve the care of women’s health conditions, today announced the launch of its first breakthrough product for preeclampsia, one of the most common and serious pregnancy complications. Encompass™ combines a novel blood test to predict personalized preeclampsia risk with a preventive action plan and a responsive virtual assistant to offer pregnant women individualized support and help them work with their care team towards the healthiest pregnancy possible.
By Mirvie · Via Business Wire · May 14, 2025

Today, Mirvie announced results of a breakthrough study published in Nature Communications, revealing new advances in the biological understanding of hypertensive disorders of pregnancy (HDP), including preeclampsia - a leading cause of maternal morbidity and mortality as well as preterm birth. Researchers used data from more than 9,000 pregnancies within the multi-center Mirvie-sponsored Miracle of Life prospective study to discover and validate RNA signatures capable of distinguishing between severe and mild hypertensive disorders of pregnancy, including preeclampsia, months before symptoms occur. The paper also validates the predictive performance of Mirvie’s simple blood test to predict preeclampsia early, at 17.5 to 22 weeks gestational age, in pregnancies without any pre-existing high-risk conditions.
By Mirvie · Via Business Wire · April 8, 2025

Mirvie, a pioneer in bringing a personalized, predictive, and preventive approach to serious pregnancy complications, today announced at the Society for Maternal-Fetal Medicine annual meeting that the Mirvie RNA platform has uncovered a unique molecular signature predictive for babies born with severe growth restriction. Unrecognized fetal growth restriction is the single largest risk factor for stillbirth.
By Mirvie · Via Business Wire · January 30, 2025

Mirvie, a company bringing a personalized, predictive, and preventive approach to pregnancy complications, today announced the company was selected to present new research demonstrating the ability of the Mirvie RNA platform to predict the risk of severe fetal growth restriction in pregnancy.
By Mirvie · Via Business Wire · January 8, 2025

As May marks Preeclampsia Awareness Month, leading maternal health experts, preeclampsia survivors and industry leaders have come together on a new campaign, “Preeclampsia Prevention is Possible.” The campaign elevates awareness of a major paradigm shift underway focused on the prevention of the disease, encouraging the use of evidence-based strategies so pregnant patients and providers can actively work together to create the healthiest pregnancy possible.
By Mirvie · Via Business Wire · May 1, 2024

Mirvie, a company pioneering the prediction of life-threatening pregnancy complications months in advance, today announced the completion of enrollment of its landmark 10,000 person research study for pregnancy health, in collaboration with leading experts in obstetrics and maternal-fetal medicine.
By Mirvie · Via Business Wire · November 14, 2023

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced its South San Francisco-based laboratory has been certified by the Centers for Medicare and Medicaid Services (CMS) under the Clinical Laboratory Improvement Amendments (CLIA) to perform high-complexity clinical testing.
By Mirvie · Via Business Wire · September 26, 2023

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced a $4.6 million grant from the Bill & Melinda Gates Foundation to support the company’s efforts to predict unexpected pregnancy complications before they happen by revealing the underlying biology of each pregnancy for women on a global scale. Specifically, the funding will help launch study sites in Cameroon, Ghana, and Zambia to understand preeclampsia among pregnant women in low- and middle-income countries (LMIC) using the Mirvie RNA platform via a simple blood test from the mom.
By Mirvie · Via Business Wire · August 2, 2023

A new special report published in the American Journal of Obstetrics and Gynecology (AJOG) provides a groundbreaking approach to preeclampsia, one of the most pressing issues in maternal health today and will translate the prediction of risk into prevention of disease.
By Mirvie · Via Business Wire · April 28, 2023

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced the findings of the first ever “Future of Pregnancy Health” Report. The report’s actionable insights to improve pregnancy health in the United States are urgently needed. Maternal mortality is on the rise and hypertensive disorders of pregnancy, including preeclampsia, are leading causes of death and have doubled between 2007 and 2019.1
By Mirvie · Via Business Wire · November 8, 2022

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced the appointment of Alison Cowan, M.D., M.S.C.R. as Head of Medical Affairs. Dr. Cowan will guide Mirvie’s continued clinical and commercial development of the proprietary Mirvie RNA platform, which is first to predict preeclampsia and preterm birth months before they happen by revealing the underlying biology of each pregnancy.[1],[2]
By Mirvie · Via Business Wire · August 30, 2022

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced it has raised a total of $90 million to date following the close of its Series B funding. The financing was led by Decheng Capital with additional funding from funds and accounts managed by BlackRock, Foresite Capital, General Catalyst, GV, Khosla Ventures, and Mayfield as well as a debt facility with Comerica Bank. The funding will support Mirvie’s continued clinical and commercial development of the proprietary Mirvie RNA platform, which is the first to predict preeclampsia and preterm birth months before they happen by revealing the underlying biology of each pregnancy.[1],[2]
By Mirvie · Via Business Wire · May 17, 2022

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced the company received U.S. Food and Drug Administration (FDA) Breakthrough Device designation for its test to indicate a woman’s individualized risk of developing preeclampsia before symptoms occur. The test is powered by the proprietary Mirvie RNA platform, which is designed to predict the risk of unexpected complications by revealing the underlying biology of each pregnancy.[1], [2]
By Mirvie · Via Business Wire · May 3, 2022

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced the publication of a study in the American Journal of Obstetrics and Gynecology, a leading obstetrics and gynecology medical journal. The results demonstrate the Mirvie RNA platform is first to predict preterm birth by revealing the underlying biology of each pregnancy. The breakthrough research shows the platform can predict the condition months before symptoms occur and identify distinct biological pathways driving its development.
By Mirvie · Via Business Wire · April 13, 2022

Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced data presented at the Society for Maternal-Fetal Medicine 42nd Annual Pregnancy Meeting (SMFM 2022) demonstrates the Mirvie RNA platform is first to predict preterm birth by revealing the underlying biology of each pregnancy. The breakthrough research shows the platform can predict the condition months before symptoms occur and identify distinct biological pathways driving its development.
By Mirvie · Via Business Wire · February 4, 2022
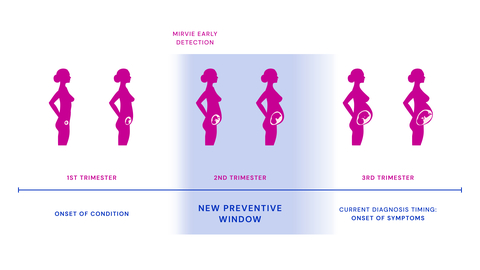
Mirvie, a pioneer in predicting unexpected pregnancy complications, today announced the publication of a study in Nature proving the proprietary Mirvie RNA platform is first to predict unexpected complications by revealing the underlying biology of each pregnancy. The research analyzed the largest and most diverse dataset of maternal transcriptomes to date. The platform opens a new window into pregnancy health for women to act and their doctors to intervene before unexpected complications become a crisis.
By Mirvie · Via Business Wire · January 5, 2022
