Articles from Endologix LLC

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, is pleased to announce that R. Scott Huennekens has been appointed to the Endologix Board of Directors.
By Endologix LLC · Via Business Wire · July 9, 2025

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced that 1,000 patients have now been treated with Percutaneous Transmural Arterial Bypass (PTAB) using the DETOUR System. This milestone includes patients in the DETOUR1 and DETOUR2 clinical studies as well as those treated post-commercial launch (July 2023).
By Endologix LLC · Via Business Wire · March 20, 2025

Endologix LLC, a privately held global medical device company specializing in innovative therapies for vascular disease, is pleased to announce the appointment of Andrew Davis as its new Chief Commercial Officer, effective immediately.
By Endologix LLC · Via Business Wire · March 3, 2025

Endologix LLC, a privately held global medical device company specializing in innovative therapies for vascular disease, today announced a leadership transition. Dr. Matt Thompson will step down as President and Chief Executive Officer and take on a new role as Executive Vice President and Chief Medical Officer. Dr. John Liddicoat has been appointed as the new President and CEO, effective immediately.
By Endologix LLC · Via Business Wire · January 7, 2025

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced the final 36-month results of the DETOUR2 Study. The DETOUR System offers a unique approach to treating complex peripheral arterial disease (PAD), enabling physicians to percutaneously bypass lesions in the superficial femoral artery, by using stents routed through the femoral vein to restore blood flow to the leg. The DETOUR System is comprised of the ENDOCROSS™ device and TORUS™ stent grafts. The DETOUR2 Study findings highlight the durable efficacy of the DETOUR System, which is comparable to open bypass with a synthetic graft. Additionally, the low rates of complications and deep venous thrombosis (DVT) demonstrate the favorable safety profile of this novel technique.
By Endologix LLC · Via Business Wire · November 5, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced that Matt Thompson, President and CEO, will present at the 22nd Annual Global Morgan Stanley Conference. Fireside chat is scheduled for September 4, 2024, at 11:30 am.
By Endologix LLC · Via Business Wire · September 3, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, is pleased to announce the appointment of Graham Phillips as its new Chief Operating Officer, effective immediately.
By Endologix LLC · Via Business Wire · June 18, 2024
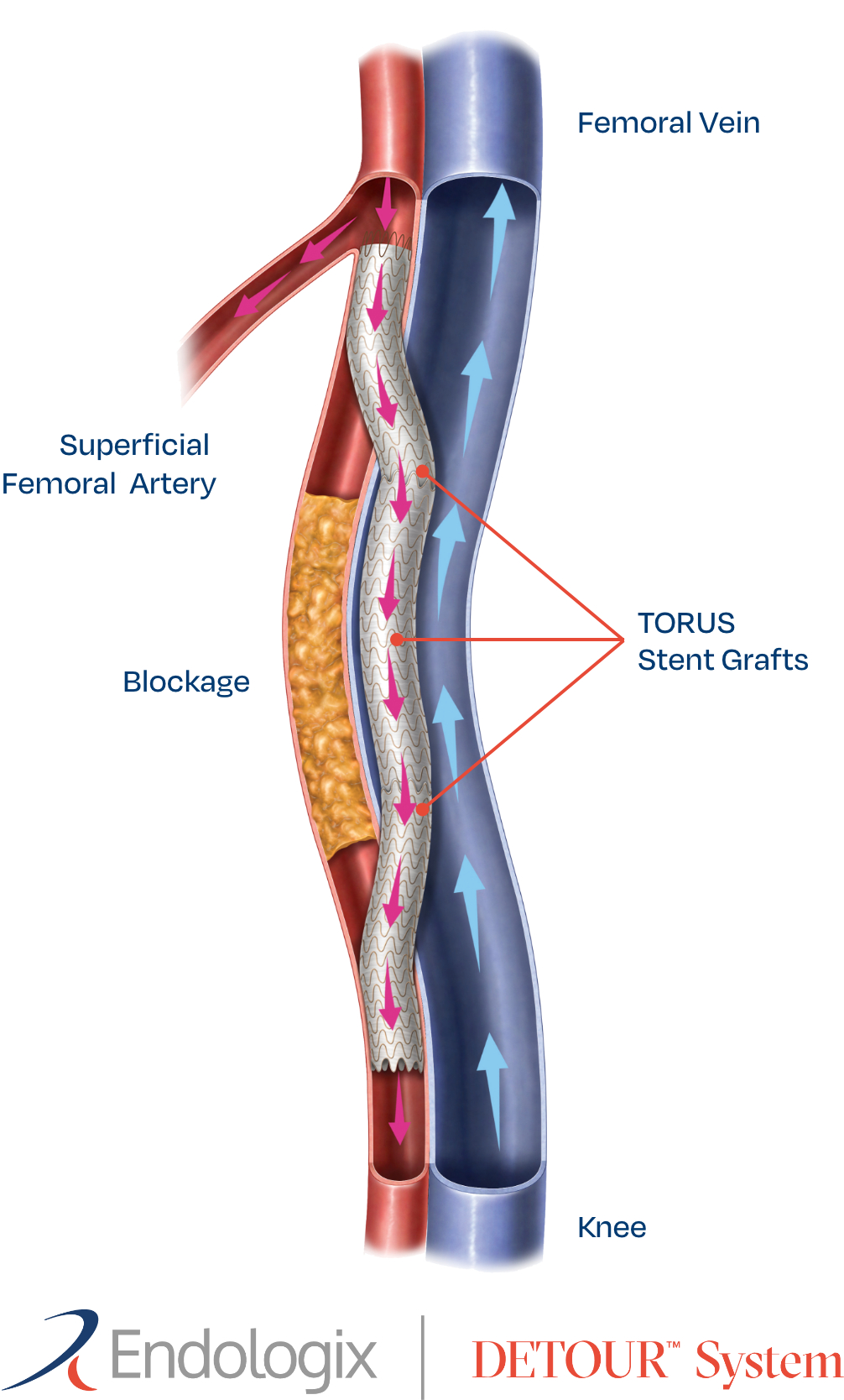
Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, has announced the online publication of the one-year results of the DETOUR2 Trial in the Journal of Vascular Surgery ( JVS1). The study demonstrated that the DETOUR System offers a viable endovascular option for patients with long segment, complex superficial femoral artery (SFA) disease. Percutaneous Transmural Arterial Bypass (PTAB) with the DETOUR System offers a novel approach to bypass the SFA using the vein as a conduit. The study highlighted low rates of complications, deep venous thrombosis (DVT), and clinically-driven target lesion revascularization (CD-TLR), and excellent one-year primary patency.
By Endologix LLC · Via Business Wire · May 13, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced that Matt Thompson, President and CEO, will present at the Life Science Intelligence (LSI) USA '24 Emerging Medtech Summit. Scheduled for March 20, 2024, at 10:55 am PT on Track 3, Thompson will offer insights into the company's innovative portfolio and its impact on vascular disease treatment.
By Endologix LLC · Via Business Wire · March 14, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced the appointment of Mike Mathias as the company’s Chief Commercial Officer, effective immediately. With a distinguished 30-year career in leading commercial organizations within the cardiovascular arena, Mike brings a wealth of experience and a proven track record of success to Endologix.
By Endologix LLC · Via Business Wire · March 5, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today the initiation of the PTAB1 Post-Market Study. This study marks the beginning of a comprehensive post-market study aimed at evaluating the real-world performance of the DETOUR System in patients undergoing treatment for long complex superficial femoral artery (SFA) disease. The study will leverage the Vascular Quality Initiative (VQI) registry infrastructure developed and supported by the Society for Vascular Surgery Patient Safety Organization (SVS PSO).
By Endologix LLC · Via Business Wire · February 29, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, is pleased to announce that Art Taylor has been appointed to the Endologix Board of Directors.
By Endologix LLC · Via Business Wire · January 25, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, is pleased to announce that Scott Ward has been appointed to the Endologix Board of Directors.
By Endologix LLC · Via Business Wire · January 9, 2024

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announces that the U. S. Centers for Medicare & Medicaid Services (CMS) granted a Transitional Pass-Through (TPT) Payment for the DETOUR System, effective January 1, 2024.
By Endologix LLC · Via Business Wire · December 27, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that the company will be presenting in two upcoming healthcare conferences:
By Endologix LLC · Via Business Wire · November 9, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced results from a pooled analysis of DETOUR1 and DETOUR2 Studies evaluating Percutaneous Transmural Arterial Bypass (PTAB) with the DETOUR System.
By Endologix LLC · Via Business Wire · November 2, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, proudly announces recent awards and nominations for its groundbreaking DETOUR System.
By Endologix LLC · Via Business Wire · October 24, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announces that the U. S. Centers for Medicare & Medicaid Services (CMS) granted a New Technology Add-on Payment (NTAP) for the DETOUR System, an FDA-designated Breakthrough Device, as part of its Fiscal Year 2024 Hospital Inpatient Prospective Payment System. NTAP was created to facilitate patient access for qualifying new medical technologies that substantially improve the diagnosis or treatment of Medicare beneficiaries. Beginning October 1, 2023, CMS will provide hospitals with additional device reimbursement when the DETOUR System is used for eligible cases in the hospital inpatient setting.
By Endologix LLC · Via Business Wire · September 7, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, is pleased to announce that Lucas Buchanan has been appointed to the Endologix Board of Directors.
By Endologix LLC · Via Business Wire · August 24, 2023
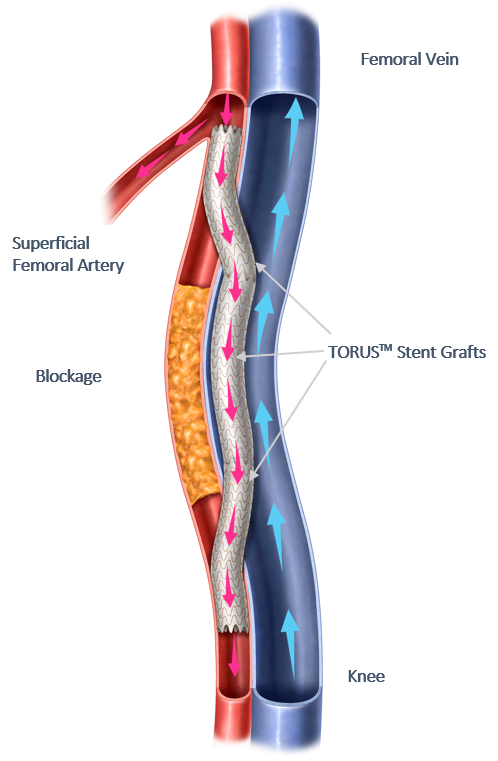
Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that the first patients underwent Percutaneous Transmural Arterial Bypass (PTAB) using the DETOUR system, since FDA approval of the system was granted. This marks the official start of its U.S. targeted market release.
By Endologix LLC · Via Business Wire · July 18, 2023
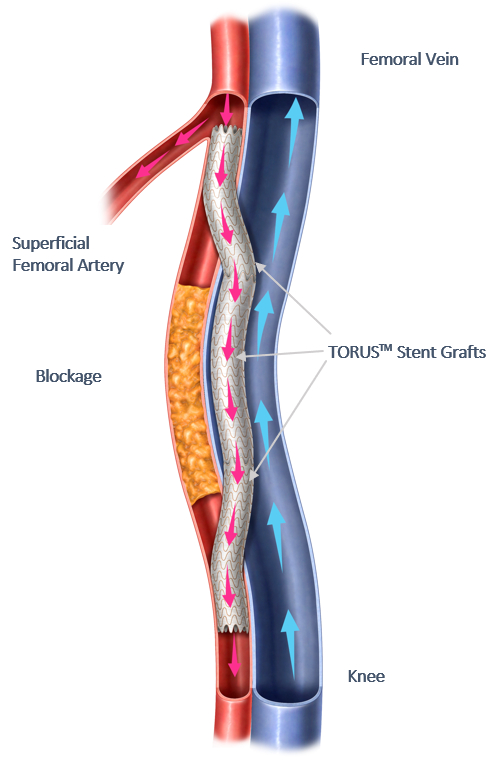
Endologix LLC, a privately held, global medical device company, dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced the 24-month results of the DETOUR2 Study. Percutaneous Transmural Arterial Bypass (PTAB) with the DETOUR™ System, recently received PMA Approval from the FDA on June 7, 2023. This system offers a unique approach to treating complex peripheral arterial disease (PAD), enabling physicians to percutaneously bypass lesions in the superficial femoral artery, by using stents routed through the femoral vein to restore blood flow to the leg. The DETOUR System is comprised of the ENDOCROSSTM device and TORUSTM stent grafts.
By Endologix LLC · Via Business Wire · June 15, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that the U.S. Food and Drug Administration (FDA) has granted approval for the DETOUR System to treat patients with complex peripheral arterial disease (PAD).
By Endologix LLC · Via Business Wire · June 8, 2023

Endologix LLC, a privately held, global medical device company, dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced the completion of the 150th case in the JAGUAR Study. This randomized controlled trial is evaluating Endologix’s ALTO Abdominal Stent Graft System, comparing its effectiveness to other commercially available endovascular aneurysm repair (EVAR) devices in the treatment of abdominal aortic aneurysms (AAA).
By Endologix LLC · Via Business Wire · May 23, 2023

Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that its DETOUR™ System has been selected as the winner of the “Medical Device Engineering Breakthrough” award in the 7th annual MedTech Breakthrough Awards. This program is conducted by MedTech Breakthrough, an independent market intelligence organization that recognizes the top companies, technologies and products in the global health and medical technology market.
By Endologix LLC · Via Business Wire · May 4, 2023
Endologix LLC, a privately held global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, has announced the online publication of the final five-year results of the LEOPARD Trial in the Journal of Vascular Surgery ( JVS1). The study's findings showed that there was no significant difference in aneurysm-related outcomes between patients randomized to the AFX endograft system, with anatomical fixation, and commercially available endografts with proximal fixation.
By Endologix LLC · Via Business Wire · April 27, 2023
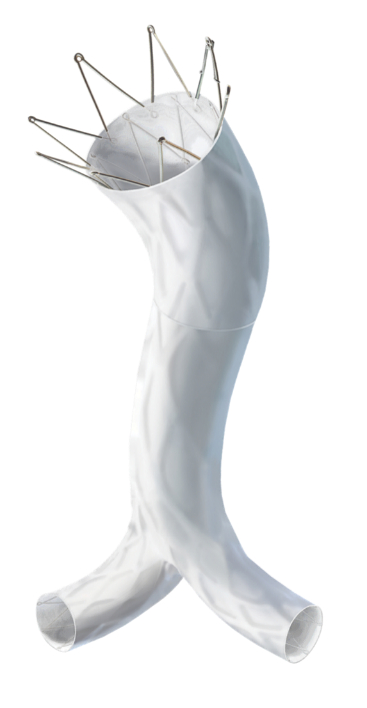
Endologix LLC, a privately held global medical device company, dedicated to improving patients’ lives with innovative interventional treatments for vascular disease, today announced that it has received U.S. Food and Drug Administration (FDA) approval for a pre-market approval (PMA) supplement relating to the AFX2 System.
By Endologix LLC · Via Business Wire · December 7, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing disruptive therapies for the interventional treatment of vascular disease, announced the 12-month results of the DETOUR 2 trial during a late-breaking clinical trial session at the 2022 VIVA Vascular InterVentional Advances (VIVA) Conference in Las Vegas, Nevada.
By Endologix LLC · Via Business Wire · November 11, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing disruptive therapies for the interventional treatment of vascular disease, announced the five-year results from the LEOPARD randomized controlled trial. LEOPARD was a prospective multi-center trial designed to directly compare the anatomically fixated AFX®2 Endovascular AAA System and the predecessor AFX device, to commercially available endografts with proximal fixation.
By Endologix LLC · Via Business Wire · November 3, 2022
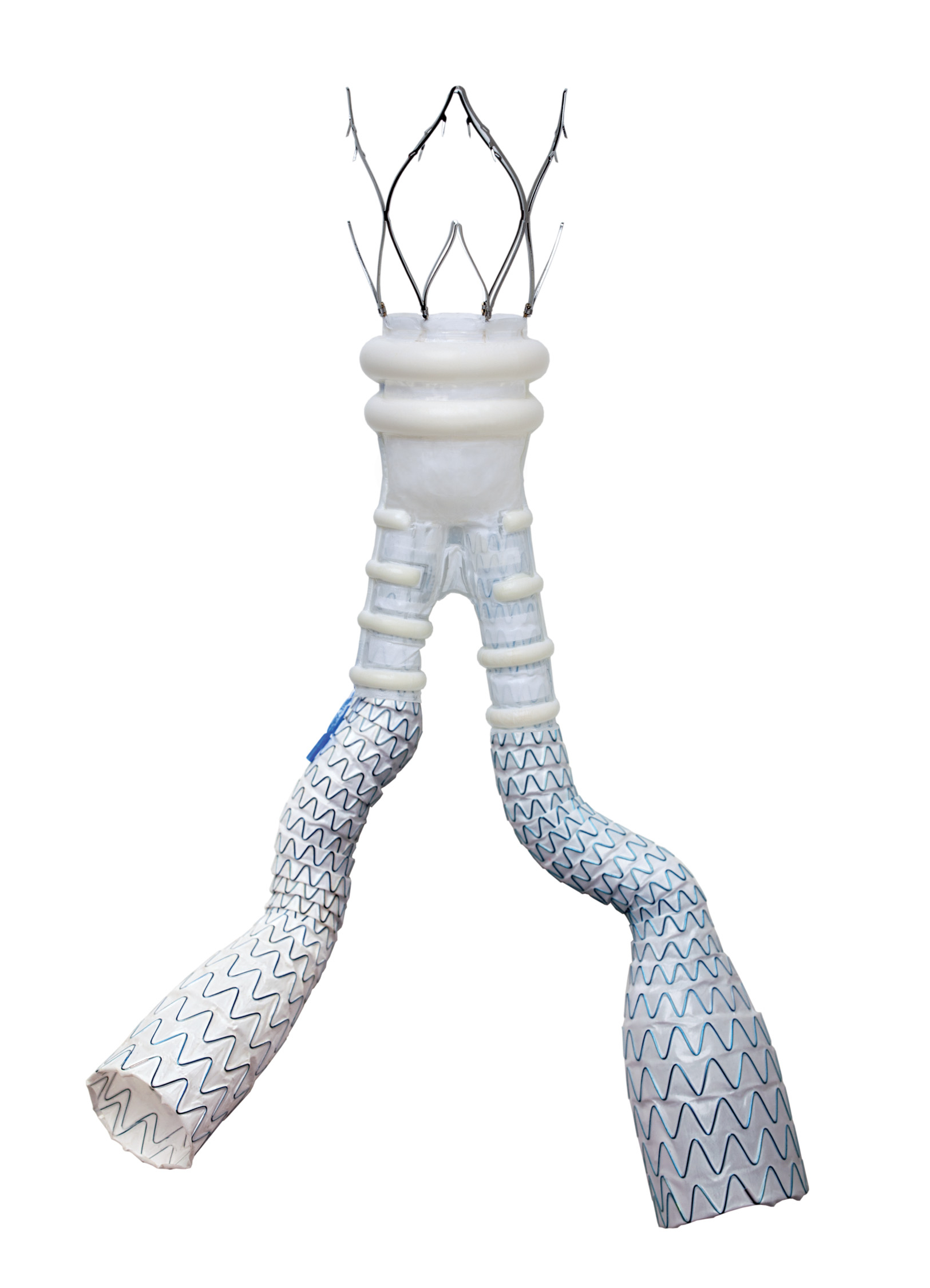
Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing disruptive therapies for the interventional treatment of vascular disease, announced that the ELEVATE (Expanding Patient Applicability with Polymer Sealing Ovation ALTO Stent Graft) IDE Study has been published online in the Journal of Vascular Surgery. The study reports the clinical outcomes of the ALTO Abdominal Stent Graft System and highlights the device’s safety and effectiveness at one-year post-procedure. Additionally, the results will be presented by the study’s principal investigator, Dr. Sean Lyden, Chairman of the Department of Vascular Surgery at Cleveland Clinic, at the VEITHsymposium in November 2022.
By Endologix LLC · Via Business Wire · October 20, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing disruptive therapies for the interventional treatment of vascular disease, today announced the submission of a Premarket Approval (PMA) application requesting approval for the DETOUR System to the U.S. Food and Drug Administration (FDA).
By Endologix LLC · Via Business Wire · October 10, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives with innovative interventional treatments for vascular disease, announced that is has received CE Mark Certification under the new EU Medical Devices Regulation [EU-MDR [(Regulation (EU) 2017/745)] for its AFX2 Endovascular AAA System. The EU-MDR is a regulation for medical devices applied by the European Commission, released in 2017 and effective as of May 26, 2021. It replaces the Medical Devices Directive (MDD). The intent of the EU MDR is to ensure a high standard of safety and quality for medical devices that are produced in, or supplied to, member countries of the European Union.
By Endologix LLC · Via Business Wire · August 4, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives with innovative interventional treatments for vascular disease, recently announced the 12-month results from the DETOUR 2 clinical trial. DETOUR 2 was an Investigational Device Exemption (IDE) study, designed to evaluate safety and effectiveness of the DETOUR System for percutaneous bypass in the treatment of long-segment femoropopliteal disease. The DETOUR System uses the ENDOCROSS™ catheter and TORUS™ stent graft to perform a totally percutaneous femoro-popliteal bypass routed through the femoral vein.
By Endologix LLC · Via Business Wire · June 23, 2022

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing innovative therapies for the interventional treatment of vascular disease, completed enrollment in the TORUS 2 IDE clinical study (NCT04130737) in the United States. The study was designed to evaluate safety and effectiveness of the TORUS Stent Graft System in the treatment of obstructive atherosclerotic lesions of the native SFA and proximal popliteal arteries.
By Endologix LLC · Via Business Wire · November 23, 2021

Endologix LLC, a privately held global medical device company dedicated to improving patients’ lives by providing innovative therapies for the interventional treatment of vascular disease, announced today the appointment of Dr. Matthew Thompson, as President and Chief Executive Officer. Dr. Thompson will also join Endologix’s Board of Directors. Richard Mott, who previously held the CEO position on an interim basis, will return to his role as Executive Chairman of the Board of Directors.
By Endologix LLC · Via Business Wire · November 10, 2021

Endologix LLC, a leader in the treatment of vascular disease, today announced that the first patient has been enrolled in the company’s JAGUAR study to compare outcomes for the company’s ALTO® Abdominal Stent Graft System to other commercially available endovascular aneurysm repair (EVAR) devices for the treatment of abdominal aortic aneurysm (AAA).
By Endologix LLC · Via Business Wire · September 29, 2021
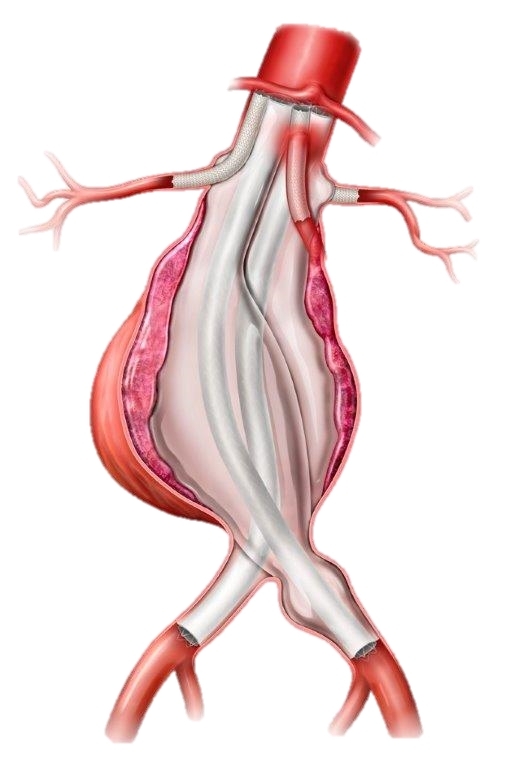
Endologix LLC, a leader in the treatment of vascular disease, today announced the company’s ChEVAS™ (Chimney EndoVascular Aneurysm Sealing) System has been granted a Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). The ChEVAS System is an investigational endovascular abdominal aortic aneurysm (AAA) sealing therapy designed to combine the Nellix® 3.5 endograft with parallel visceral stents to enable treatment of patients with juxtarenal, pararenal and suprarenal AAA.
By Endologix LLC · Via Business Wire · July 19, 2021
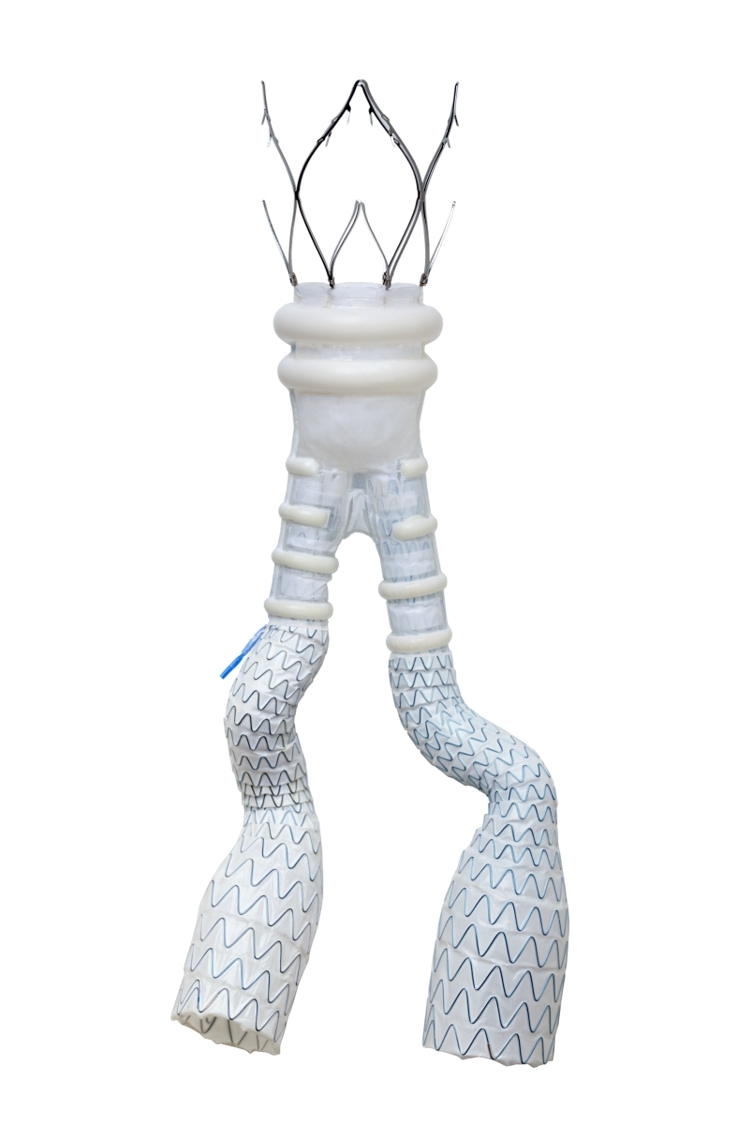
Endologix LLC, a leader in the treatment of vascular disease, today announced the first implant of its ALTO® Abdominal Stent Graft in Canada following recent approval from Health Canada. ALTO was also recently approved for commercial sale in Argentina.
By Endologix LLC · Via Business Wire · May 11, 2021

Endologix LLC, a leader in the treatment of vascular disease, today announced it has completed the acquisition of PQ Bypass, Inc., a privately held medical technology company pioneering a first-of-its-kind technology that addresses an unmet need for new treatments for severe peripheral arterial disease (PAD).
By Endologix LLC · Via Business Wire · April 13, 2021
