Articles from Cystic Fibrosis Foundation

Today, the Cystic Fibrosis Foundation announced that Adela Skenderasi, CFA, will join the organization as its next Chief Investment Officer (CIO) on October 13, 2025.
By Cystic Fibrosis Foundation · Via Business Wire · September 24, 2025

Today, the Cystic Fibrosis Foundation announced an additional investment of up to $24 million in Prime Medicine to continue the development of a gene editing therapy for people with cystic fibrosis (CF).
By Cystic Fibrosis Foundation · Via Business Wire · July 16, 2025

The Cystic Fibrosis Foundation has agreed to invest up to $15 million in ReCode Therapeutics to support their gene editing collaboration with Intellia Therapeutics. The funding is intended to be used to develop a gene editing therapy that could be delivered into the lung cells of people with cystic fibrosis.
By Cystic Fibrosis Foundation · Via Business Wire · November 18, 2024

Today, the Cystic Fibrosis Foundation announced that it will provide up to $15 million to Prime Medicine for preclinical research into gene editing for cystic fibrosis. Prime Medicine is using a gene editing technology called prime editing — a technology that enables insertions or deletions of small segments of DNA at precise sites. Prime Medicine — founded by researchers who pioneered the development of this unique editing technology — is investigating whether prime editing could treat several diseases, including CF. This technology has the potential to enable many types of CF mutations to be corrected with a single type of genetic therapy.
By Cystic Fibrosis Foundation · Via Business Wire · January 25, 2024

The Cystic Fibrosis Foundation is investing up to $9 million in additional funds in Arcturus Therapeutics to test an inhaled messenger RNA (mRNA) therapy that could treat the underlying cause of cystic fibrosis in all people living with the disease, including those with two copies of rare and nonsense mutations.
By Cystic Fibrosis Foundation · Via Business Wire · September 26, 2023

Today, the Cystic Fibrosis Foundation announced changes to its Board of Trustees, including the election of Kate O’Donnell, MBA, and Dodzie Sogah, PhD, as trustees, and Elise Eberwein, MBA, as a non-voting advisor.
By Cystic Fibrosis Foundation · Via Business Wire · August 9, 2023

The Cystic Fibrosis Foundation today announced the recipients of its eighth annual Impact Grants, a program which provides up to $10,000 in funding annually for up to two years to individuals and nonprofit organizations within the cystic fibrosis community who are leading projects to support people living with or impacted by the disease.
By Cystic Fibrosis Foundation · Via Business Wire · July 31, 2023

Today, the Cystic Fibrosis Foundation announced that Yvonne Massenburg will join the organization as its next Chief People Officer. In the role, she will be responsible for the Foundation’s overall human resources strategy, serving as a strategic advisor and business partner to senior leadership as the organization works to advance its mission and strengthen its partnership with the cystic fibrosis community.
By Cystic Fibrosis Foundation · Via Business Wire · April 5, 2023

The Cystic Fibrosis Foundation recently agreed to provide up to $15.5 million to Anagram Therapeutics (formerly known as Synspira Therapeutics) to conduct early-stage clinical trials of a novel enzyme replacement therapy.
By Cystic Fibrosis Foundation · Via Business Wire · April 4, 2023

Today, the Cystic Fibrosis Foundation announced that Kathryn Brown will join the organization as its next Chief Communications and Marketing Officer. She will oversee strategic communications and marketing across the Foundation, including initiatives to inform and engage the cystic fibrosis community, as it accelerates its mission to find a cure for the progressive, rare, genetic disorder.
By Cystic Fibrosis Foundation · Via Business Wire · March 29, 2023

The Cystic Fibrosis Foundation will invest up to $15 million in ReCode Therapeutics for the development of its messenger RNA (mRNA) therapy that could eventually provide a treatment option for all people with cystic fibrosis regardless of their mutations. The CF Foundation joins other institutional and strategic investors who participated in ReCode’s Series B financing, totaling $210 million.
By Cystic Fibrosis Foundation · Via Business Wire · January 10, 2023

Today, the Cystic Fibrosis Foundation announced that Irena Barisic has been appointed the next Executive Vice President, Chief Operating and Financial Officer. Ms. Barisic will transition from her current role as Executive Vice President, Chief Financial and Administrative Officer, a position she has held since June 2021.
By Cystic Fibrosis Foundation · Via Business Wire · December 6, 2022

The Cystic Fibrosis Foundation today announced its investment of $6 million in Carbon Biosciences to support the company‘s preclinical research into an innovative gene therapy approach for cystic fibrosis. Carbon Biosciences is the first company to publicly launch from the Foundation’s multi-million-dollar collaboration with Longwood Fund, an early stage venture capital firm creating and investing in science-based companies that develop novel solutions to treat important unmet medical needs, and is part of the Foundation’s Path to a Cure initiative.
By Cystic Fibrosis Foundation · Via Business Wire · June 21, 2022

The Cystic Fibrosis Foundation today announced its investment of $5 million in Sionna Therapeutics, a company founded in 2019. The company is focused exclusively on developing cystic fibrosis transmembrane conductance regulator (CFTR) modulators, a type of therapy that treats the underlying cause of CF.
By Cystic Fibrosis Foundation · Via Business Wire · April 19, 2022

Today, the Cystic Fibrosis Foundation announced its first-ever “Golden Ticket” Competition to find the next promising treatment for cystic fibrosis in a collaboration with Bakar Labs, the incubator at the University of California, Berkeley’s Bakar BioEnginuity Hub. Researchers developing new technologies in gene editing, gene delivery, and gene therapy/gene insertion that have potential application to cystic fibrosis are invited to apply for the competition starting May 2. Up to three winners will receive one year of free laboratory space and support at Bakar Labs to help advance their transformative work.
By Cystic Fibrosis Foundation · Via Business Wire · April 18, 2022

Today, the Cystic Fibrosis Foundation announced an award to Eloxx Pharmaceuticals Inc. for up to $15.9 million in additional funding to support the expansion of its ongoing ELX-02 clinical program focused on developing therapies for people with CF who have nonsense mutations.
By Cystic Fibrosis Foundation · Via Business Wire · March 29, 2022

The Cystic Fibrosis Foundation today announced that its Board of Trustees has unanimously elected KC White, an adult living with cystic fibrosis, as the next Chair of the Board of Trustees, succeeding long-time Chair Catherine C. “Cam” McLoud, who has served in the role since 1999. The election of White marks the first time in the Foundation’s history that the Board will be led by a person with CF, a historic milestone in the fight against a disease once considered solely pediatric.
By Cystic Fibrosis Foundation · Via Business Wire · February 22, 2022

Today, the Cystic Fibrosis Foundation announced that Steven Rowe, MD, will be its next chief scientific officer. In this role, Dr. Rowe will oversee the Foundation’s basic science, academic research, and venture philanthropy program investments, which include the CF Foundation Lab and the Path to a Cure initiative.
By Cystic Fibrosis Foundation · Via Business Wire · January 26, 2022

The Cystic Fibrosis Foundation announced today that it has invested in SalioGen Therapeutics to support the company’s preclinical research into a novel genetic therapy for cystic fibrosis. SalioGen’s Gene CodingTM approach is designed to turn on, turn off, or modify the function of any gene in the genome.
By Cystic Fibrosis Foundation · Via Business Wire · January 5, 2022

The Cystic Fibrosis Foundation announced today that it has awarded up to $5 million to BiomX Inc. to conduct a Phase 1b/2a clinical trial to test the safety and tolerability of bacteriophage (phage therapy) in Pseudomonas aeruginosa infections in people living with cystic fibrosis.
By Cystic Fibrosis Foundation · Via Business Wire · January 4, 2022

Today, the Cystic Fibrosis Foundation announced its investment of $3.5 million in Pulmocide Limited, a U.K.-based biotech company, to develop an inhaled drug (opelconazole) to prevent Aspergillus fungal infections in lung transplant recipients. The investment is part of the CF Foundation’s Lung Transplant Initiative, a commitment to improve outcomes and support people with CF throughout the transplant journey. It also marks the CF Foundation’s first funding of a potential treatment specifically for people who have received a lung transplant.
By Cystic Fibrosis Foundation · Via Business Wire · December 6, 2021

Today, the Cystic Fibrosis Foundation announced a first-of-its-kind collaboration with Pioneering Medicines, an initiative of Flagship Pioneering, to spur the development of genetic-based therapies for cystic fibrosis. As part of its Path to a Cure research agenda, the Foundation expects to invest up to $110 million in the collaboration, with an initial commitment of $20 million and the option to invest additional funds as promising therapies arise. Through this agreement, Pioneering Medicines will combine technologies from several Flagship-founded companies to develop a separate company specifically focused on potential treatments for cystic fibrosis.
By Cystic Fibrosis Foundation · Via Business Wire · November 3, 2021

The Cystic Fibrosis Foundation today announced the recipients of its sixth annual Impact Grants, which provide up to $10,000 of funding per year for up to two years to individuals and nonprofit organizations within the cystic fibrosis community who are leading projects to support people living with the disease.
By Cystic Fibrosis Foundation · Via Business Wire · July 26, 2021
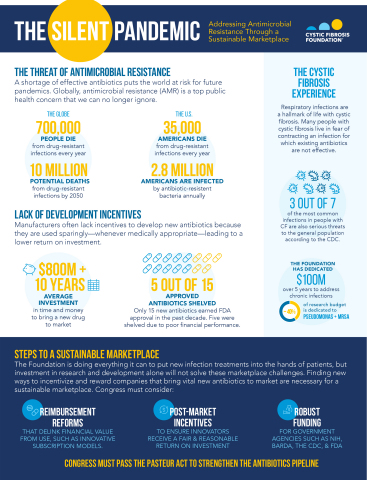
Today, the Cystic Fibrosis Foundation affirmed its support for the PASTEUR Act, a bipartisan proposal that, if passed, will support the development of new antibiotics and promote appropriate use of existing ones. The organization issued the following statement:
By Cystic Fibrosis Foundation · Via Business Wire · June 16, 2021

Today, the Cystic Fibrosis Foundation announced the initiation of the HERO-2: Home Reported Outcomes Study, a first-of-its-kind, at-home observational study that aims to characterize the range of outcomes people experience while using Trikafta® (elexacaftor/tezacaftor/ivacaftor). The study will rely on observations that individuals living with cystic fibrosis make about their health in their daily lives to understand the impact of Trikafta when taken in the real world.
By Cystic Fibrosis Foundation · Via Business Wire · June 2, 2021

Today, the Cystic Fibrosis Foundation announced three new research awards as part of its Path to a Cure initiative to accelerate the development of treatments for the underlying cause of cystic fibrosis for every person with the disease. The latest round of funding includes up to $2.6 million to Eloxx Pharmaceuticals to identify compounds that could potentially treat individuals who have nonsense mutations, as well as awards to Hunterian Medicine and Metagenomi to advance genetic therapies for all people with CF.
By Cystic Fibrosis Foundation · Via Business Wire · May 27, 2021

Today, the Cystic Fibrosis Foundation announced that it will invest up to $8.4 million in SpliSense’s Series B funding round to develop an antisense oligonucleotide (ASO) therapy for people with cystic fibrosis who have splicing mutations and potentially other rare mutations. The investment is part of the Foundation’s Path to a Cure, a research agenda to accelerate treatments for the underlying cause of cystic fibrosis for every person with CF.
By Cystic Fibrosis Foundation · Via Business Wire · May 13, 2021
